Exufiber in practice
The condition of peri-wound skin improved and diabetic foot ulcers reduced in size in a study of 21 patients treated with Exufiber®.
Open, non-comparative, multi-centre post clinical study of the performance and safety of a gelling fibre wound dressing on diabetic foot ulcers
Chadwick, P., McCardle, J. Journal of Wound Care 2016; 25(4):290-300.
- Foot ulceration in diabetic patients is difficult to manage.
- Patients whose diabetes is poorly managed are at an increased risk of foot ulceration and foot amputation.
- Dressing selection is one important aspect of diabetic foot ulcer (DFU) management.
- DFUs are associated with medical costs, as well as personal, social and economic costs.
- Like other chronic wounds, DFUs may produce excessive levels of exudate; as a result, the periwound skin is at risk of maceration.
Aims
To evaluate the performance and safety of Exufiber, a gelling fibre wound dressing, in the treatment of DFUs.
Methods
- This was an open, non-comparative multi-centre investigation.
- Both in and out-patients were eligible.
Primary study objective:
- To evaluate the performance and safety of Exufiber when used as intended on DFUs.
- In line with this objective, a number of evaluation parameters were measured to monitor the condition of the peri-wound skin (evaluated in terms of changes from baseline assessments): maceration; redness/irritation; rash/eczema; blistering; dermatitis; skin stripping; trauma to the wound edges; product degradation on the skin.
Secondary study objectives:
- Evaluation of dressing-related pain (measured using a 100mm visual analogue scale (VAS)).
- Wound status (measured by changes in wound size and healing phase).
- Clinician/patient opinions of the test product.
- Technical performance of the test product (measured by the presence of dressing residue following removal, and handling of wound exudate).
- Patients with more than one ulcer were eligible for inclusion into the investigation, however only one ulcer per patient was included in the investigation.
- Each patient was treated according to the local clinical routine and all dressings were applied according to the manufacturer’s instructions.
- Patients participated in the investigation until complete wound healing or if the treated wound became dry (whereby the test product was no longer applicable) or for 12 weeks, whichever occurred first.
- Patients were assessed at baseline and again at weeks 1, 2, 4, 6, 8 and 12 post-treatment.
- Dressing changes were performed according to the local clinical routine (usually 3 times per week) when the dressing was saturated and depending on the status and location of the wound; dressing changes between visits were allowed.
- At each assessment visit, the following variables were assessed:
- Wound size, volume (using the Pictzar program) and depth at the deepest point of the wound (using a q-tip); measurements made every fourth week and an additional measurement at week 6)
- Appearance of ulcer bed
- Condition of peri-wound skin
- Technical performance
- Amount and type of wound exudate
- Need for debridement
- Adverse events (AEs)/adverse device effects (ADEs)
- Pain (before removal of the secondary dressing; before removal of the test product; during removal of the test product; after removal of the test product). Some patients were not eligible for inclusion in the pain assessment due to neuropathy
- Clinical signs of infection
Results
- 21 patients were included in the investigation, from two centres.
- All of the patients recruited (eligible for treatment with the test product) were Caucasian males, with an active DFU; the mean patient age was 59.9 years.
Peri-wound skin condition
- The number of patients with healthy/intact periwound skin increased from 6 patients (28.6%) at baseline to 14 patients (66.7%) at the final visit.
- The percentage of patients with a given sign of poor peri-wound skin status decreased or remained at zero for the majority of signs, from baseline to the final visit.
- There were no occurrences of product degradation on the skin.
Wound exudate
- There was a steady decrease in wound exudate volume throughout the study period; the percentage of patients without wound exudate increased from 0% at baseline to 33.3% at week 12.
- Wound exudate was mostly serous throughout the study period.
Pain
- Pain levels were very low throughout the study period (some patients were not eligible for inclusion in the pain assessment due to neuropathy or completion of ulcer healing).
Wound status
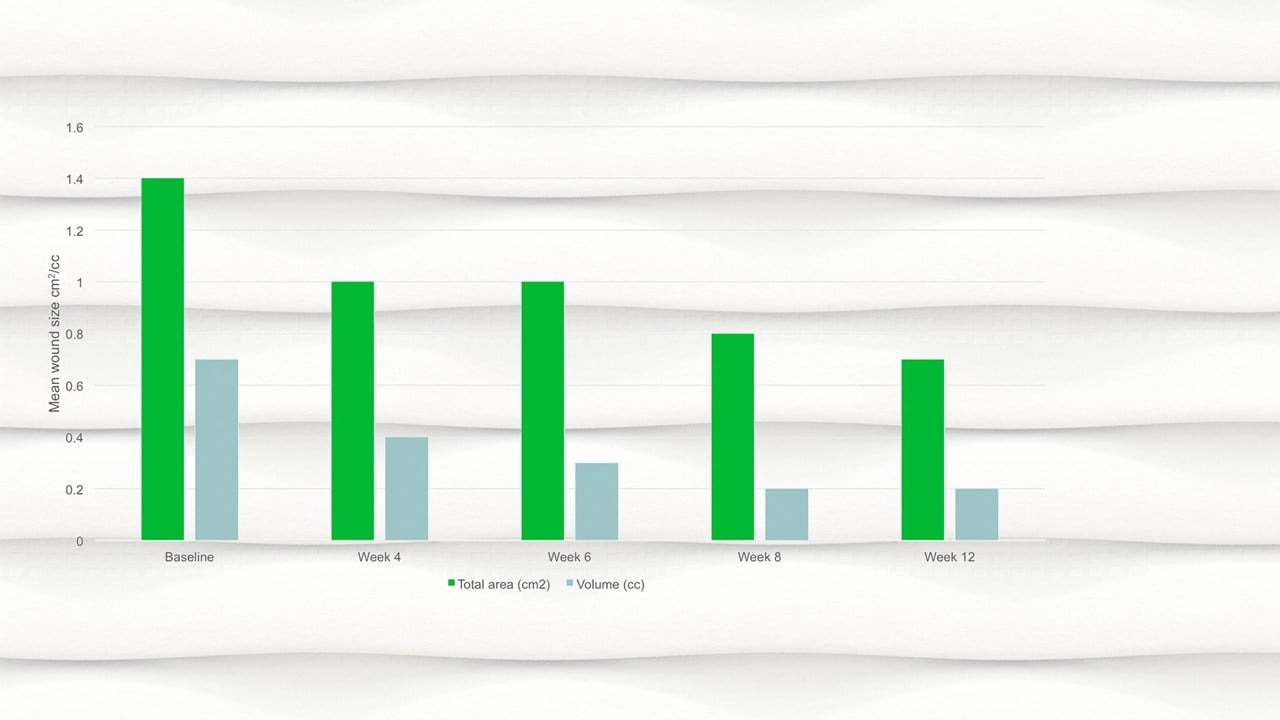
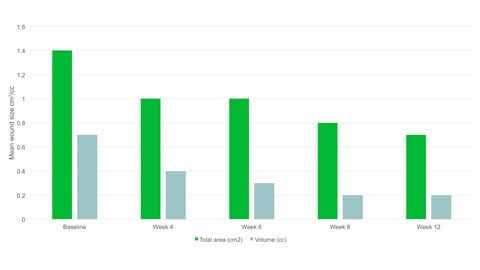
- By week 12 the mean wound area had halved compared to baseline, with a mean area of 0.7cm2.
- There was a significant reduction in wound area, p=.094 and wound volume, p=.0056 from baseline to the final visit.
- There was a gradual increase in the mean percentage of epithelialisation tissue and a small decline in the mean percentage of granulation tissue throughout the study; the percentage of non-viable tissue remained low throughout the study.
- The number of healed wounds increased from 1 (4.8%) at week 1 to 5 (23.8%) at week 12.
Clinical signs of infection
There was a low level of clinical signs of infection throughout the study period.
Investigator/nurse evaluation
The dressing under evaluation was rated highly in terms of ease of application, ease of removal, lack of adherence to the wound bed and healthy intact skin at removal, flexibility, conformability, ability to absorb exudate, ability to retain slough and blood and overall experience.
Patient evaluation
The dressing under evaluation was rated highly in terms of lack of anxiety during dressing change, ease of movement whilst wearing the dressing, ability of the dressing to remain in place during wear, lack of stinging or burning whilst wearing the dressing and comfort during wear.
There were no reported AEs/ADEs during the course of the study that were judged to be related to the investigational product.
Conclusions
- The results from this study have demonstrated the ability of the investigational wound dressing to minimise damage to the peri-wound skin and dressing-associated pain.
- Although most wounds remained unhealed at the end of the study period, improvements were noted in terms of tissue type and a significant reduction in wound area and volume.
- The dressing’s technical performance was demonstrated by an ability to absorb and retain exudate.
- Product safety was demonstrated by an increase in the number of patients with healthy/intact periwound skin and the lack of identified productrelated AEs/ADEs.










